An Introduction Bispecific Antibody Therapeutics
Bispecific antibodies, as the name suggests, are antibodies with two binding sites – directed against two different antigens, or in some cases, two different epitopes on the same antigen.
While mAbs have become mainstays in various therapies, they have several limitations – not including failed treatment responses and the development of drug resistance. Tumors are multi-factorial, with multiple signaling pathways implicated in pathogenesis, that often doesn’t allow for single-drug treatments to be as powerful a therapeutic option.
In contrast, bsAbs have been posited as having several advantages, including the redirection of specific immune cells to cancer cells and also enabling simultaneous blocking of two different pathways.
Following years of R&D, the approval of catumaxomab (anti-EpCAM and anti-CD3) and blinatumomab (anti-CD19 and anti-CD3) was a major milestone in the development of bsAbs., with four more being approved globally to date. Over 230 are in pre-clinical studies or clinical investigations.
BsAb Functionality:
Since Bispecific antibodies have two different binding sites, their functionality can interfere with multiple surface receptors or ligands. They can also place targets into close proximity, either to support protein complex formation on one cell or trigger contact between cells. Their flexible functionality allows for four main mechanisms of action:
-
Blocking immune checkpoints (a hot topic of research!)
-
Activating immune cells
-
Blocking dual signaling pathways
-
Forcing association of protein complexes
In the past, bsAbs were generated by chemical conjugation of two different, purified mAbs, or by fusing two hybridomas resulting in a quadroma cell line that produced, amongst others, bispecific IgG molecules. Of course, advances in genetic engineering supported the development of a range of recombinant bsAb formats, with over 50 formats now available.
Structure of the bsAb:
Typically, bsAbs can be divided into two major classes – those with and those without an Fc region – or, as they are referred to as – IgG-like and non-IgG-like molecules. IgG-like bsAbs that retain the Fc-mediated effector functions (such as ADCC, CDC and ADCP) also usually have longer serum half-lives owing to their larger size and FcRn-mediated recycling. On the flip side, non-IgG-like bsAbs are smaller in size, leading to better tissue penetration.
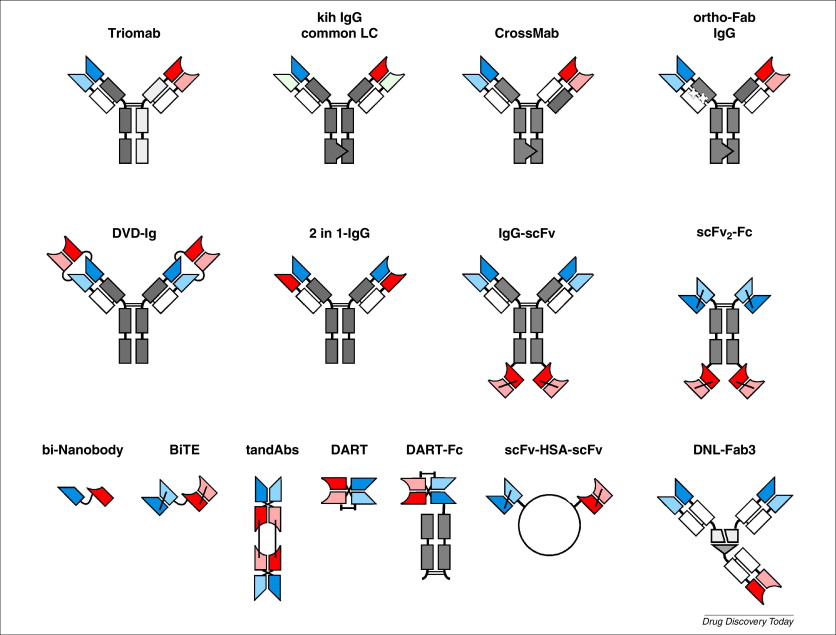
Fig. 2. Various bispecific antibodies (bsAbs) are currently in clinical development or are already approved for cancer therapy. The upper two lines depict immunoglobulin (Ig)-like bsAbs comprising an IgG Fc region, either as bivalent or tetravalent molecules. Furthermore, several small bsAb and bsAb fusion proteins have entered clinical trials. Abbreviations: BiTE, bispecific T cell engager; DART, Dual affinity retargeting; DNL, dock-and-lock; DVD-Ig, dual variable domain immunoglobulins; HSA, human serum albumin; kih, knobs into holes.
Learn more about Bispecific T Cell Engager (BiTE) antibodies and their specific impact on onco-therapies: More About BiTE Ab.
Mode of Action:
Recruitment and Activation of Immune Cells:
Bispecific antibodies bind and activate immune cells at one binding site and recognize spe- cific antigens on tumor cells at the other binding site, which mechanically recruit immune cells (e.g. T cells and NK cells) to the tumor area for killing. Examples include CD3×CD19 BsAbs, CD3×CD20 BsAbs, etc.
Blocking of Dual Signaling Pathways:
The onset and progression of diseases such as tumors usually involve multiple signals, and blocking a single signal may not completely inhibit tumor pro- gression, and sometimes cause activation of other pathways. Bispecific antibodies work by targeting two receptors on the cell surface, inhibiting or activat- ing two signals at the same time. Examples include PD-1 x CTLA-4 BsAbs, HER2 dual epitope BsAbs, etc.
Forcing Association Of Protein Complexes:
Bispecific antibodies can act on factors that are free in the body and promote the formation of protein complexes. A heterodimeric common light chain IgG connects FXIa and FX and thereby overcomes FVIII deficiency.
Clinical Relevance:
BsAbs have been shown to be clinically superior to monoclonal antibodies in their specificity and have a wide range of therapeutic applications under tumor and other diseases. Their clear advantage is the enhanced specificity, targetability and reduced off-target toxicity they allow for.
-
Redirection of T cells to tumor cells is one of the bsAb features that allows to overcome to immne escape mechanism that limits tumor specific T cell responses. bsAbs form a transient cytolytic synapse between the T cell and targeted tumor cells. The subsequent activation and proliferation of T cells leads to tumor lysis. A number of bsAbs including Triomab, BiTE, DART, and FynomAb provide new treatment options for patients.
-
Blocking dual signaling pathways offers a unique appeal over treatment options like Herceptin and Cetuximab. bsAbs allow for blocking downstream resistance mechanisms and provides more effective responses.
-
bsAbs also target tumor growth and metastasis – with multiple angiogenesis factors, dual targeting leads to superior outcomes, especially compared to single pathway inhibitors.
-
Support of protein complexation in the clotting cascade, or tumor-targeted immune cell recruiters and/or activators (forced connections) is another functionality that is being researched
What’s next?
The identification of target pairs and bsAbs with potential synergistic effects also poses a big challenge, necessitating a high-throughput approach. Moreover, immunogenicity is a complex issue in drug design and development. In clinical trials, adverse effects are often reported and hamper the success of bsAbs.
For those working with existing targets for development of new indications and biosimilars / generics, Krishgen offers a range of ELISA to quantify drug levels accurately and quickly in serum and plasma samples. Find an ELISA for pharmacokinetic studies and for anti-drug antibody research and get a quote today. Amongst others:
[2] Ma J, Mo Y, Tang M, Shen J, Qi Y, Zhao W, Huang Y, Xu Y, Qian C. Bispecific Antibodies: From Research to Clinical Application. Front Immunol. 2021 May 5;12:626616. doi: 10.3389/fimmu.2021.626616. PMID: 34025638; PMCID: PMC8131538.
[3] Chames P, Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs. 2009 Nov-Dec;1(6):539-47. doi: 10.4161/mabs.1.6.10015. PMID: 20073127; PMCID: PMC2791310.