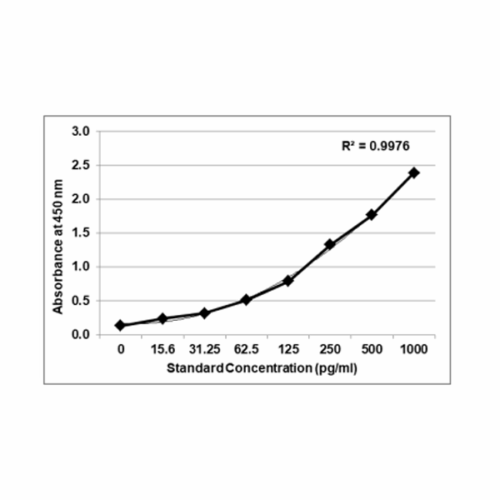
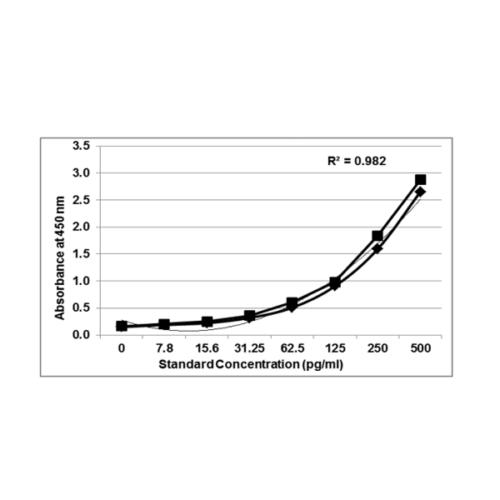
Precisionbind Cytokine ELISA for Cell and Gene Therapy
High-Performance Cytokine Quantification for Therapeutic Antibody & Cell-Gene Therapy Research
PrecisionBind Cytokine ELISA Kits are designed to deliver reliable, reproducible, and regulatory-grade cytokine measurements in serum, plasma, and cell culture supernatants.
Engineered for therapeutic mAb development, cell & gene therapy potency testing, and cytokine release studies, our kits combine superior analytical sensitivity with simplified workflows for both discovery and QC environments.

Key Highlights
Ultra-Sensitive Detection
Detect cytokines down to sub-pg/mL concentrations, enabling confident measurement even after high serum dilutions.
Validated for Real Biological Matrices
Fully validated in human serum, plasma (EDTA/heparin), cerebrospinal fluids and cell culture supernatants, ensuring accuracy across sample types.
Consistent Performance, Lot After Lot
PrecisionBind kits are manufactured under stringent quality systems, providing <20% inter-assay variability and verified lot-to-lot reproducibility.
Rapid & Robust Workflow
Ready-to-use coated plates and optimized reagents reduce assay setup time and operator variability.
Technology Advantage
PrecisionBind technology leverages high-affinity antibody pairing and optimized capture kinetics, minimizing background interference and maximizing signal-to-noise ratio.
Each kit undergoes cross-reactivity and interference testing, ensuring specificity even in complex biological matrices — crucial for cytokine quantification in therapeutic product characterization.
Complete Validation Documentation
Every kit includes performance data for LOD, LOQ, accuracy, linearity, parallelism, and stability to meet the expectations of regulated labs.
Quality Data Interpolation
Where available, our cytokine ELISA kits have standards calibrated against WHO/NIBSC calibrators for precision and accuracy.
Ideal for CGT & mAb Programs
Tailored for applications such as:
Cytokine release assays (CRS monitoring)
Potency and bioactivity testing
Lot comparability and stability studies
PK/PD and immunogenicity biomarker analysis
Cytokine Coverage
Choose from a comprehensive portfolio including:
Pro-inflammatory cytokines: IL-1β, IL-6, TNF-α, IFN-γ
Regulatory cytokines: IL-10, TGF-β
Growth & activation factors: IL-2, GM-CSF, VEGF, IL-8
Interferons: IFN-α, IFN-β
Featured Products
Why Choose PrecisionBind
Designed by scientists for scientists in biotherapeutic and CGT development
High sensitivity, low matrix interference, and true reproducibility
Validated for biological relevance — not just buffer results
Comprehensive technical support for assay transfer and validation
OEM and bulk formats available for kit developers and CROs
Applications
Cytokine release risk assessment (CAR-T, TCR, NK, MSC therapies)
Functional potency testing for neutralizing mAbs and immunomodulators
PK/PD biomarker quantitation in preclinical or clinical samples
Stability and comparability studies under ICH/FDA bioassay guidelines
Suggested Website Callouts / Taglines
You can use these as banner or section headings:
“Built for Biotherapeutics – Trusted by CGT Innovators”
“Because every picogram matters.”
“From Discovery to GMP – Precision You Can Quantify.”
“Regulatory-Ready Cytokine ELISAs for Real-World Matrices.”
1500+ Citations
Since 2010, Krishgen ELISA have been cited in over 1500+ publications from journals around the world. We hope to continue supporting researchers around the world. Find all our publications here.
Related Research Areas
Coming Soon…
Related Blogs
Coming Soon..
If you are unable to locate your desired ELISA kit on our website, or have a technical query, please drop us a message and our team will come back to you with availability. If we do not have it available, we will suggest a feasible custom development option.