Artificial Intelligence in Drug Discovery Research: A Brief Overview
Artificial intelligence, as it evolves, has become an increasingly important tool in life sciences research in recent years. The ability of machine learning algorithms to analyse large amounts of data quickly and accurately has made it possible for scientists to identify new patterns and insights in fields such as genomics, drug discovery, and clinical trials. Significant success has been achieved in a wide range of fields, such as genomics, protein folding, disease diagnosis, imaging, and clinical tasks.
There are several types of AI algorithms for life sciences research that show promise and are being explored for their specific applications.
In genomics, for example, AI algorithms can analyse large datasets of genomic data and identify previously undiscovered genetic mutations and their links to specific diseases. With great speed and accuracy in these algorithms, insights into the underlying causes of diseases and the development of more targeted treatments have been a promising field. Multi-omics refers to the integration of multiple types of omics data, such as genomics, transcriptomics, proteomics, and metabolomics. AI is being used to integrate these different types of data to provide a more comprehensive understanding of biological systems and identify new therapeutic targets.
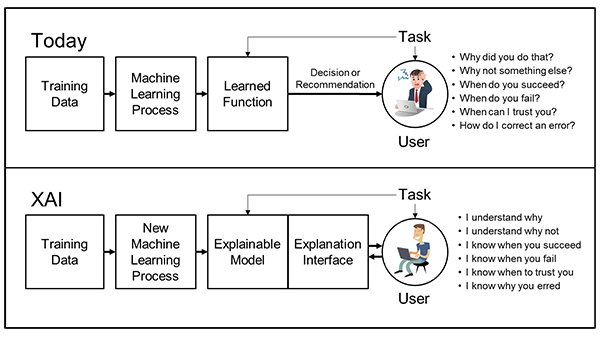
One of the major challenges in applying AI in life sciences research is the need for interpretability and transparency in AI models, because its interpretations have real-world consequences. To understand data, it is important to have a case-based reasoning approach to compare and place a given sample with respect to other examples in the dataset. Thus, Explainable AI (XAI) is an emerging field that aims to address this challenge by developing AI models that can provide clear and understandable explanations of their outputs and decision-making processes.
Reinforcement learning and predictive analytics algorithms have also shown immense promise in optimize drug design and identify new drug targets. They are also being developed to identify potential drug targets and predict the efficacy and safety of new drug candidates.
In drug discovery research and development particularly, various kinds of AI algorithms are helping speed up the process of identifying potential new drugs. Involvement of AI in the development of a pharmaceutical product from the bench to the bedside can be predictably imagined given that it can aid rational drug design, assist in decision making; determine the right therapy for a patient, including personalized medicines; and manage the clinical data generated and use it for future drug development. Through detailed analysis of increasingly complicated and specific drug compounds and their mechanisms, AI algorithms can predict the effectiveness and safety of new drug candidates. This helps reduce the time and cost involved in the early stages of drug development, particularly for combination therapies and studies of repurposed drugs. According to market research firm Bekryl, AI has the potential to offer over US$70 billion in savings for the drug discovery process by 2028.
So how is AI being applied to drug discovery and development?
1. Predicting the toxicity of drug candidates:
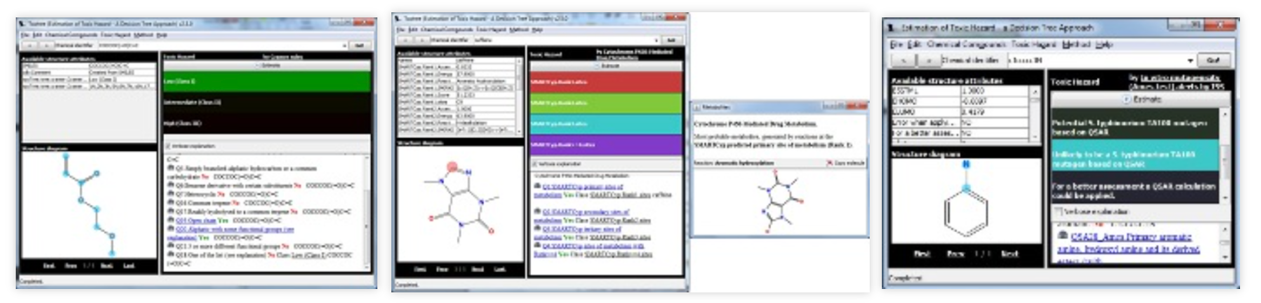
The prediction of the toxicity of any drug molecule is vital to avoid toxic effects. Several web-based tools, such as LimTox, pkCSM, admetSAR, and Toxtree, are available to help reduce the cost. Advanced AI-based approaches look for similarities among compounds or project the toxicity of the compound based on input features. This has helped speed up the drug discovery process by identifying promising candidates more quickly and reducing the need for time-consuming and expensive experimental testing.
The Tox21 Data Challenge organized by the National Institutes of Health, Environmental Protection Agency (EPA), and US Food and Drug Administration (FDA) was an initiative to evaluate several computational techniques to forecast the toxicity of 12 707 environmental compounds and drugs. An ML algorithm named DeepTox outperformed all methods by identifying static and dynamic features within the chemical descriptors of the molecules, such as molecular weight (MW) and Van der Waals volume and could efficiently predict the toxicity of a molecule based on predefined 2500 toxicophoric features.
2. Identifying new drug targets:
AI can analyse vast amounts of biological and chemical data to identify new drug targets that were previously unknown. By analysing gene expression data, protein interactions, and other biological information, AI algorithms can help researchers to identify the underlying mechanisms of diseases and develop new therapies that target those mechanisms.
AI can also assist in structure-based drug discovery by predicting the 3D protein structure because the design is in accordance with the chemical environment of the target protein site, thus helping to predict the effect of a compound on the target along with safety considerations before their synthesis or production. The AI tool, AlphaFold, which is based on DNNs, was used to analyze the distance between the adjacent amino acids and the corresponding angles of the peptide bonds to predict the 3D target protein structure and demonstrated excellent results by correctly predicting 25 out of 43 structures.
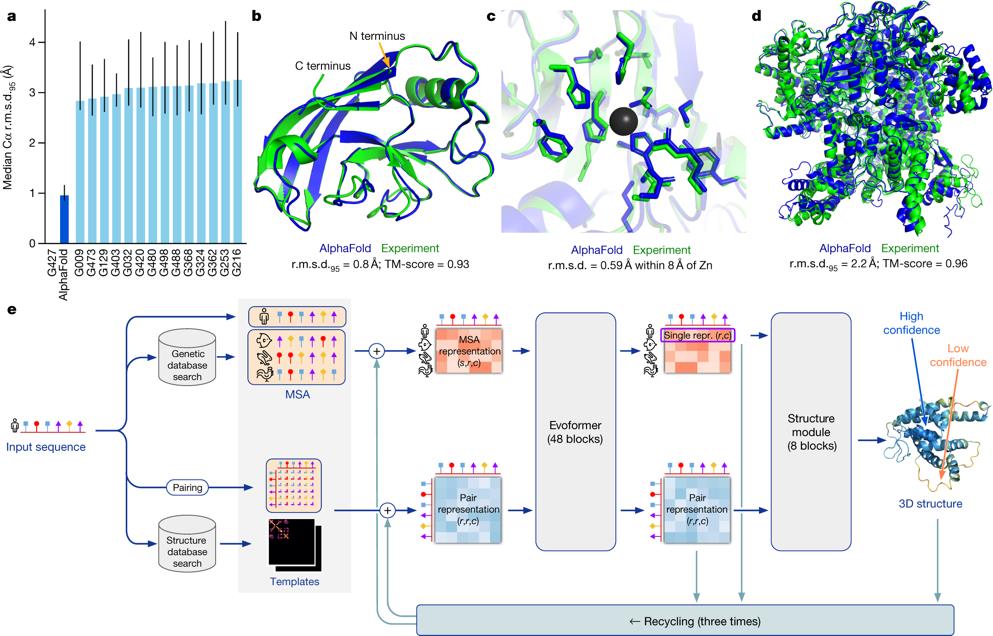
Image Reference: Jumper, J., Evans, R., Pritzel, A. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). https://doi.org/10.1038/s41586-021-03819-2
3. Optimising drug development:
AI can be used to optimize the drug development process by predicting which patients are most likely to benefit from a particular treatment. By analysing large datasets of patient information, AI algorithms can identify patient subgroups that are more likely to respond to a particular therapy, allowing for more targeted clinical trials. Decision-support tools use rule-based systems to select the type, nature, and quantity of the excipients depending on the physicochemical attributes of the drug and operate through a feedback mechanism to monitor the entire process and intermittently modify it.
4. Predicting adverse drug reactions:
The efficacy of drug molecules depends on their affinity for the target protein or receptor. Drug molecules that do not show any interaction or affinity towards the targeted protein will not be able to deliver the therapeutic response. In some instances, it might also be possible that developed drug molecules interact with unintended proteins or receptors, leading to toxicity. Hence, drug target binding affinity (DTBA) is vital to predict drug–target interactions. AI-based methods can measure the binding affinity of a drug by considering either the features or similarities of the drug and its target. Feature-based interactions recognize the chemical moieties of the drug and that of the target to determine the feature vectors. By contrast, in similarity-based interaction, the similarity between drug and target is considered, and it is assumed that similar drugs will interact with the same targets.
AI can also be used to predict adverse drug reactions based on patient data and drug interactions. By analysing large datasets of patient information and drug interactions, AI algorithms can predict which patients are at higher risk of adverse reactions and identify potential safety issues with new drugs.
5. Virtual screening:
The process of discovering and developing a drug can take over a decade and costs US$2.8 billion on average. Even then, nine out of ten therapeutic molecules fail Phase II clinical trials and regulatory approval. AI algorithms are being used to perform virtual screening of chemical compounds, which involves predicting the activity of a compound against a specific target. By using machine learning algorithms to analyse large amounts of chemical data, researchers can identify potential new drug candidates that are more likely to be effective against a specific disease. Algorithms, such as Nearest-Neighbour classifiers, RF, extreme learning machines, SVMs, and deep neural networks (DNNs), are used for VS based on synthesis feasibility and can also predict in vivo activity and toxicity.
6. Drug repurposing:
AI is being used to identify new uses for existing drugs, which can save time and reduce costs in drug development. Drug–protein interactions have a vital role in the success of a therapy. The prediction of the interaction of a drug with a receptor or protein is essential to understand its efficacy and effectiveness, allows the repurposing of drugs, and prevents polypharmacology. Repurposing an existing drug qualifies it directly for Phase II clinical trials. This also reduces expenditure because the cost of relaunching an existing drug (?US$8.4 million) compared with the launch of a new drug entity (?US$41.3 million) is vastly reduced. Additionally, it allows for a faster development-to-market timeline.
Logistic regression platforms, such as PREDICT, SPACE, and other ML approaches, consider drug–drug, disease–disease similarity, the similarity between target molecules, chemical structure, and gene expression profiles while repurposing a drug.
Image referencfe: Ching T et al., Opportunities and obstacles for deep learning in biology and medicine. J R Soc Interface. 2018 Apr;15(141):20170387. doi: 10.1098/rsif.2017.0387. PMID: 29618526; PMCID: PMC5938574.
While the use of AI in life sciences research has many potential benefits, there are also challenges that need to be addressed. One of the biggest challenges is the need for high-quality data to train machine learning algorithms. In many cases, data is scattered across different databases and may not be standardised, making it difficult for algorithms to learn effectively.
Another challenge is the need for transparency and interpretability. These algorithms can sometimes produce results that are difficult to understand or explain, which can make it difficult for scientists to use the insights effectively.
Despite these challenges, the potential benefits of AI in life sciences research are significant. As machine learning algorithms become more sophisticated and data quality improves, machine learning will accelerate drug discovery and reduce attrition rates, ultimately making more novel drugs available to patients, faster.
References:
(1) Sanjoy Dey, Prithwish Chakraborty, Bum Chul Kwon, Amit Dhurandhar, Mohamed Ghalwash, Fernando J. Suarez Saiz, Kenney Ng, Daby Sow, Kush R. Varshney, Pablo Meyer. Human-centered explainability for life sciences, healthcare, and medical informatics.
Patterns, Volume 3, Issue 5, 2022,100493, ISSN 2666-3899,
(2) Bharath Ramsundar, Peter Eastman, Patrick Walters, Vijay Pande, Deep Learning for the Life Sciences, 2019.
(3) Ching T, Himmelstein DS, Beaulieu-Jones BK, Kalinin AA, Do BT, Way GP, et al. Opportunities and obstacles for deep learning in biology and medicine. J R Soc Interface. 2018;15:20170387. PMID:29618526
(4) https://bekryl.com/industry-trends/ai-artificial-intelligence-in-drug-discoverymarket-size-analysis
(5) Duch W. Artificial intelligence approaches for rational drug design and discovery. Curr. Pharm. Des. 2007;13:1497–1508.
(6). Blasiak A. CURATE. AI: optimizing personalized medicine with artificial intelligence. SLAS Technol. 2020;25:95–105.
(7) Mak K.-K., Pichika M.R. Artificial intelligence in drug development: present status and future prospects. Drug Discovery Today. 2019;24:773–780
(8) Álvarez-Machancoses Ó, Fernández-Martínez J.L. Using artificial intelligence methods to speed up drug discovery. Expert Opin. Drug Discovery. 2019;14:769–777.
(9) Fleming N. How artificial intelligence is changing drug discovery. Nature. 2018;557 S55–S55.
(10) Öztürk H. DeepDTA: deep drug–target binding affinity prediction. Bioinformatics. 2018;34:i821–i829.
(11) Wan F., Zeng J. Deep learning with feature embedding for compound–protein interaction prediction. bioRxiv. 2016;2016
(12) Persidis A. The benefits of drug repositioning. Drug Discov. World. 2011;12:9–12.
(13) Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekade RK. Artificial intelligence in drug discovery and development. Drug Discov Today. 2021 Jan;26(1):80-93. doi: 10.1016/j.drudis.2020.10.010. Epub 2020 Oct 21. PMID: 33099022