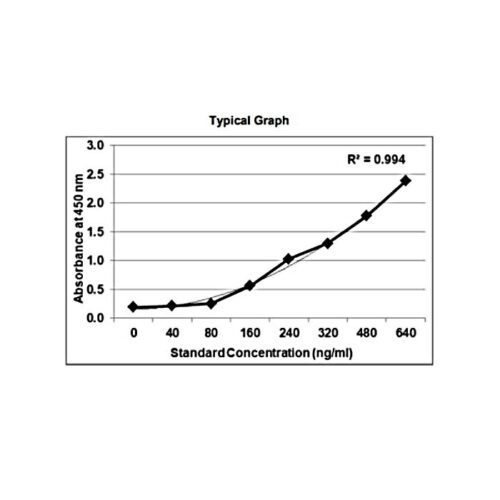
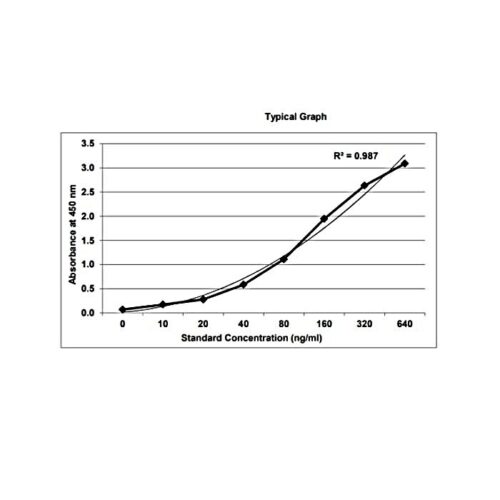
Background:
Bevacizumab is a recombinant Human IgG kappa monoclonal antibody specific for all human vascular endothelial growth factor A (VEGF-A) isoforms. An angiogenesis inhibitor, it acts by selectively binding circulating VEGF, thereby inhibiting the binding of VEGF to its cell surface receptors. This inhibition leads to a reduction in microvascular growth of tumor blood vessels and thus limits the blood supply to tumor tissues. These effects also lower tissue interstitial pressure, increase vascular permeability, may increase delivery of chemotherapeutic agents, and favor apoptosis of tumor endothelial cells. Additionally, Bevacizumab binds to and neutralizes all human VEGF-A isoforms an bioactive proteolytic fragments, but not mouse or rat VEGF.
Intended Use:
For Estimation of Bevacuzimab (AVASTIN) in human serum and plasma. Please note the kit has not been optimized to be used for any other animal species. Should you require a specific sera tested, please connect with us at sales@krishgen.com for optimization.
Principle:
The method employs the quantitative sandwich enzyme immunoassay technique. Antibodies to Bevacizumab are pre-coated onto microwells. Samples and standards are pipetted into microwells and human Bevacizumab present in the sample are bound by the capture antibody. Then, a HRP (horseradish peroxidase) conjugated anti-Bevacizumab antibody is pipetted and incubated. After washing microwells in order to remove any non-specific binding, the ready to use substrate solution (TMB) is added to microwells and color develops proportionally to the amount of Bevacizumab in the sample. Color development is then stopped by addition of stop solution. Absorbance is measured at 450 nm.
![]() If you have published a paper by using any of our ELISA since 01/01/2023, kindly fill out the “Krishgen Publication Reward Application Form” with complete information and send it by at email: info@krishgen.com, with the subject “Krishgen Publication Reward”. We will get back to you with the Amazon / Krishgen Credit Reward after we confirm it ASAP!
If you have published a paper by using any of our ELISA since 01/01/2023, kindly fill out the “Krishgen Publication Reward Application Form” with complete information and send it by at email: info@krishgen.com, with the subject “Krishgen Publication Reward”. We will get back to you with the Amazon / Krishgen Credit Reward after we confirm it ASAP!