The GENLISA Human Interferon Gamma (IFNgamma / IFNg) ELISA includes features like:
– Ready to use protocol with break-apart wells for ease of use
– Standardisation and High Reproducibility
– Lot to Lot Consistency
– Accuracy and Precision
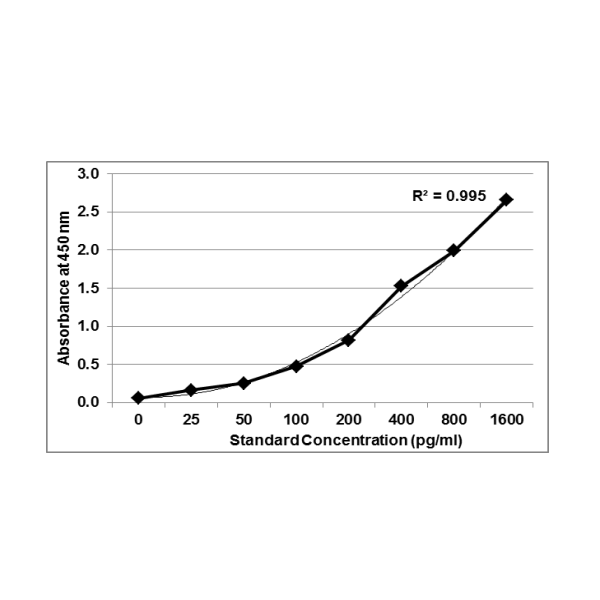
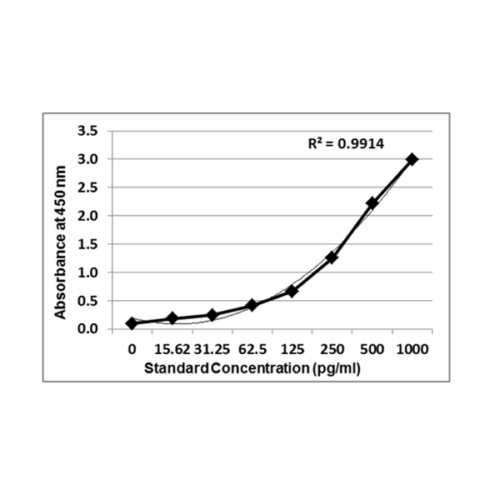
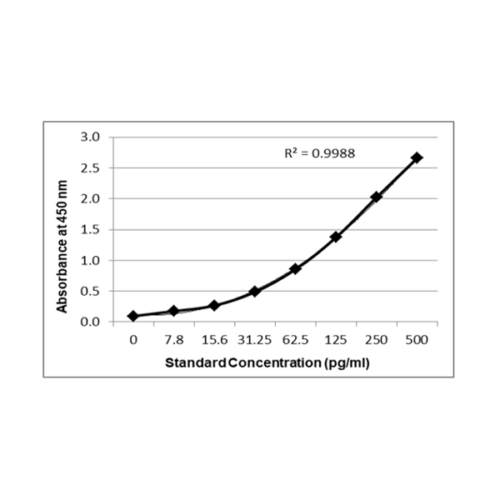
Principle Of Assay: This ELISA is a sandwich immunoassay. Antibodies are coated on 96 well plates. The antigen protein present in sample and standard respectively bind to the coated wells. The wells are washed and an antibody:HRP Conjugate is added which binds to the bound complex in the well. Washing is performed to remove any unbound material. TMB substrate is added and the enzyme reaction is stopped by dispensing of stop solution into the wells. The optical density (OD) of the solution at 450 nm is directly proportional to the amount of antigen protein present in the standard or samples.
Disclaimer: The data indicated herein with specifications are changed from time to time at time of production of the assay. We request you to confirm the specifications including the assay range and procedure as per the most current IFU (instructions for use) accompanying the assay kit.
Validated against seven points for a GOLD RING Standard Quality ELISA – the benchmark sign for Krishgen Quality. The The GENLISA ELISA kits are used for assessing the specific biomarker in samples analytes which may be human serum, plasma, biological fluids and cell culture supernatant. The kit uses indirect sandwich assay with double antibodies – capture and detection to ensure a high degree of sensitivity and specificity in the estimation of Interferon Gamma (IFNgamma / IFNg);