Introduction: A variety of proteins which are used as therapeutic agents in humans and animals are produced through recombinant expression in VERO cells. The manufacturing and purification process of these products tends to leave the potential for contamination by Host Cell Proteins (HCPs) from VERO cells, which may result in adverse toxic or immunological reactions that ultimately affect the efficacy of the therapeutic agent. The simple, objective and semi-quantitative ELISA is a highly sensitive method that aids in purification process development, process control, quality control and product release testing optimally. Intended Use: This generic kit is intended in determining the presence Vero Host Cell Proteins contamination in various products that are manufactured through recombinant expression in VERO cells. The kit has been validated successfully for testing of in process and final product HCPs in variety of products regardless of growth and purification process.
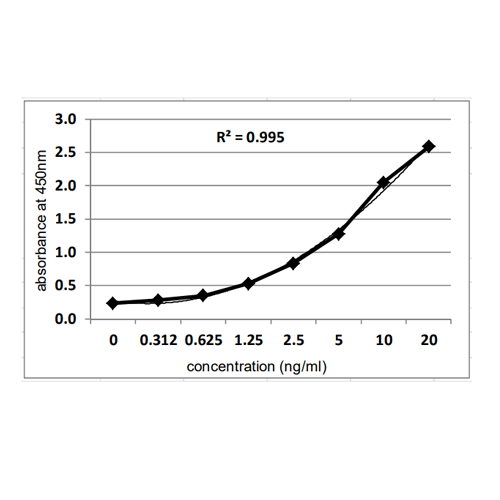
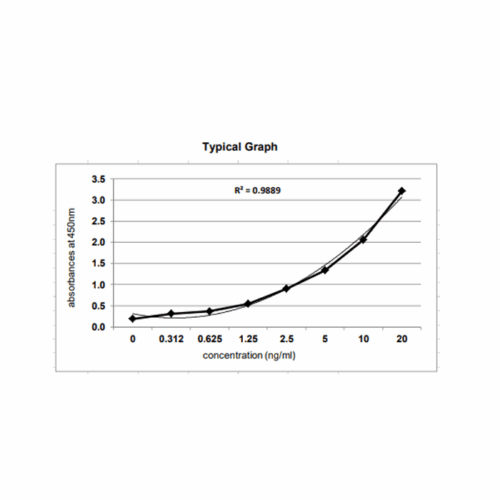
Principle: The method employs sandwich ELISA technique. Monoclonal antibodies are pre-coated onto microwells. Samples and standards are pipetted into microwells and Vero HCP present in the sample are bound by the antibodies. Biotin labeled antibody is added and followed by Streptavidin-HRP is pipetted and incubated to form a complex. After washing microwells in order to remove any non-specific binding, the substrate solution (TMB) is added to microwells and color develops proportionally to the amount of Vero HCP in the sample. Color development is then stopped by addition of stop solution. Absorbance is measured at 450 nm.